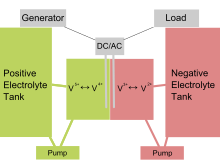
Operation of the Redox Flow Battery
The redox flow battery is a promising solution for renewable energy storage. Unlike traditional batteries, it operates on the principle of ion exchange between two tanks containing liquid electrolytes. These electrolytes, rich in metal ions, allow energy to be stored and released through redox reactions.
The operation of this battery relies on several key components:
- Two electrolyte reservoirs: Each contains a different solution, usually vanadium ions of different valences (V2+/V3+ for the anode and VO2+/VO2+ for the cathode).
- An ion exchange membrane: It allows the passage of ions between the two reservoirs while physically separating the solutions to prevent their mixing.
- Two electrodes: Placed on either side of the membrane, they facilitate the redox reactions necessary to store or release energy.
- Pumps and piping: They ensure the circulation of electrolytes between the reservoirs and the electrodes.
During charging, electricity powers the system, initiating chemical reactions that store energy in chemical form. During discharge, the process reverses; the chemical reactions generate electricity that can then be used.
This type of battery offers several notable advantages:
- Scalability: Storage capacity can be easily increased by enlarging the size of the electrolyte tanks.
- Long lifespan: The separation of storage and energy conversion components minimizes material wear, thus providing a longer lifespan.
- Safety: Using non-flammable liquids, these batteries are less prone to the risks of spontaneous combustion.
The redox flow battery represents a flexible and sustainable energy storage technology, perfectly suited to the current needs of renewable energies.
Key Components of the Battery
A redox flow battery is a type of electrochemical energy storage that uses reduction-oxidation (redox) reactions to convert chemical energy into electrical energy. This system is often used for large capacity applications, such as grid-scale energy storage, due to its flexibility and extended cycle capability. Redox flow batteries are distinguished by the use of two electrolyte solutions containing dissolved chemical species that circulate between two separate reservoirs.
The operation of the redox flow battery is based on the principle of separation between the electrochemical assembly and the storage reservoir. When the battery is charged, the electrolytes containing metal ions are pumped into an electrochemical cell where the redox reaction occurs. These electrolytes, often composed of vanadium, are separated by an ion-permeable membrane to avoid cross-contamination. During discharge, the reverse chemical reactions produce a flow of electrons that generates usable electricity.
The key components of the redox flow battery can be divided into several essential parts:
- Electrolyte reservoirs: contain the electrochemical solutions. There are usually two, each containing a different type of electrolyte (oxidizer and reducer).
- Pumping system: allows the circulation of electrolytes through the electrochemical cell.
- Electrochemical cell: where the redox reactions occur. It contains the electrodes and the ion-permeable membrane.
- Permeable membrane: separates the two compartments of the electrochemical cell, preventing electrolyte mixing while allowing the passage of ions.
In summary, the redox flow battery presents a promising solution for large-scale energy storage due to its unique characteristics, including the flexibility of size and capacity, and the ability to recharge simply by replacing the electrolytes.
The Energy Conversion Process
The redox flow battery is an innovative solution for energy storage, particularly suited for stationary applications. It relies on electrochemical mechanisms to store and release energy. Unlike conventional batteries, it uses liquid electrolytes stored in separate tanks and allows dissociation of power from storage capacity.
To operate, the redox flow battery circulates electrolytes through an electrochemical cell where the redox reaction takes place. This reaction is reversible, allowing the battery to be charged and discharged multiple times without significant material degradation.
The energy conversion process in a redox flow battery is divided into several steps:
- The electrolytes, containing the oxidizing and reducing chemical species, are stored in external tanks.
- During charging, the electrolytes are pumped into a cell where oxidation and reduction reactions occur, thus storing energy in chemical form.
- The stored energy is then released during discharge, reversing the chemical reactions to produce an electrical current.
This type of battery has several advantages, including great flexibility in terms of scalability and maintenance, due to the separation of energy and power. Furthermore, their lifespan is generally longer compared to traditional batteries.
Redox flow batteries offer a promising alternative to integrate more renewable energy sources into electrical grids, thereby contributing to a more sustainable future.
Articles similaires
Thank you!
We will contact you soon.